Attenuation of Seizure Susceptibility Through Chemo-Genetic Mobilization of Parvalbumin Interneuron Inhibitory Reserve
Abstract number :
3.049
Submission category :
1. Basic Mechanisms / 1D. Mechanisms of Therapeutic Interventions
Year :
2018
Submission ID :
501857
Source :
www.aesnet.org
Presentation date :
12/3/2018 1:55:12 PM
Published date :
Nov 5, 2018, 18:00 PM
Authors :
Ervin L. Johnson, Boston Children's Hospital; Sarika Gurnani, Boston Children's Hospital; Sameer C. Dhamne, Boston Children's Hospital; Henry Lee, Boston Children's Hospital; Takao K. Hensch, Boston Children's Hospital, Harvard University; and Alexander R
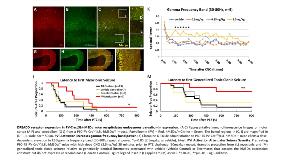
Rationale: Most current anti-seizure drugs target ligand- or voltage-gated membrane channels to inhibit excitatory signaling, facilitate inhibitory signaling or generally dampen neuronal activity. Despite the availability of >20 drugs that take this approach, up to one-third of patients with epilepsy cannot achieve adequate seizure control. Here we describe an alternative approach in which we directly stimulate parvalbumin-positive inhibitory interneuron (PVIs) activity using the designer receptors exclusively activated by designer drugs (DREADD) system. Methods: To achieve PVI-specific DREADD receptor expression, mice that conditionally-express the activating DREADD receptor, hM3Dq (LSL-hM3Dq), were bred with mice that express Cre recombinase in PVIs (PV-Cre). Susceptibility to the chemoconvulsant Pentylenetetrazole (PTZ) was tested after pretreatment with the DREADD ligand Clozapine-N-Oxide (CNO) or vehicle. Littermate controls were evaluated as well. In complementary experiments, AAV-loaded with conditional hM3Dq expression constructs were injected into the cortex of PV-Cre mice. Results: Consistent with the well-established role of PVIs in regulating cortical oscillations, CNO administration to hM3Dq x PV-Cre mice increases spectral power in the gamma frequency band (p<0.05, n=9). Interestingly, CNO also induces a hypomotor state (p<0.01, n=7) and dramatically attenuates susceptibility to PTZ (p<0.01, n=7). Similar protection from PTZ result when hM3Dq is specifically expressed in cortical PVIs using the AAV delivery method (n=3, p<0.05). Conclusions: This proof-of-concept study indicates that PVIs possess significant inhibitory reserve at baseline that can be mobilized to decrease seizure susceptibility. Funding: NIH NINDS R01 NS088583 (PI: Rotenberg) NIH NINDS 1R01NS080565 (co-PIs: Hensch, Jensen)