Expanding the Allelic Spectrum in atp1a3-related Disorders with 3 Novel Mutations and Clinic Features: A Case Series
Abstract number :
2.405
Submission category :
18. Case Studies (case reports and small series less than 5 subjects will not be accepted)
Year :
2022
Submission ID :
2204107
Source :
www.aesnet.org
Presentation date :
12/4/2022 12:00:00 PM
Published date :
Nov 22, 2022, 05:23 AM
Authors :
Suad Alyamani, MD – King Faisal Specialist Hospital and Research Centre;
Rationale: Our small-cohort observations confirm that ATP1A3 mutations express a wide range of phenotypes, usually including some degree of cognitive-behavioral dysfunction (100% of patients), seizures (85% of patients), and AHC (71% of patients). Moreover, they further expand the evolving allelic spectrum of these disorders by identifying 3 novel mutations.
Methods: This was a retrospective study of 7 patients positive for ATP1A3 mutation.
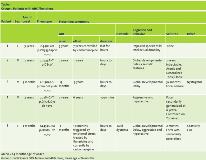
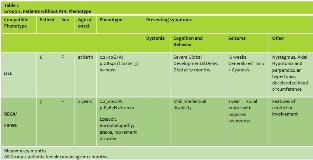
Results: Five females and 2 males had onset ages of 0–3 years (mean = 18 months). All had some degree of intellectual dysfunction, 6 had seizures (85%), 4 had neurologic abnormalities, 1 had autistic features, and one had dystonia. Group 1 consisted of 3 females and 2 males, with mean onset age of 24 months (Table 1). The hemiplegic episodes remitted in all of them except one (patient 5) who had the worst neurological impairment in this group, either spontaneously or after low-dose carbamazepine or flunarizine therapy. Two had mild neurologic abnormalities. Group 2 consisted of 2 females with mean onset age of 12 months (Table 2). One presented at birth with severe cognitive impairment and neurologic abnormalities, a phenotype compatible with EIEE (but no seizures), and died of apnea at 17 months old. The other presented at 2 years old with mild cognitive impairment, neurologic abnormalities, seizures, and a phenotype compatible with RECA. Notably, MRI was unremarkable in 6 patients and in one patient (patient 6) thinking of Corpus Callosum (Figure 1) Consanguinity present in 70% of parents but no positive family history of similar cases in the families, the genotype differed in all 7 patients, and 3 previously unreported de novo mutations appeared (Patients 1, 3, and 5).
Conclusions: The clinical features in this small cohort concur with previous reports indicating that ATP1A3 mutations cause a wide range of phenotypes, frequently including intellectual dysfunction, seizures, and neurologic abnormalities. Although it is not possible to generalize from only 7 patients, the results may suggest that patients with AHC might tend to present later and have milder neurological signs than patients without AHC, and that they usually show resolution of hemiplegic episodes spontaneously or after anticonvulsant treatment and in one patient persistence of AHC seem to be associated with severe neurological dysfunction. The fact that both Group 2 patients were female while the Group 1 sex distribution was approximately equal does not necessarily indicate a gender predilection. In agreement with previous reports of genotypic variability, every patient had a different genetic abnormality, and we discovered 3 new mutations. Our small-cohort observations confirm that ATP1A3 mutations express a wide range of phenotypes, usually including some degree of cognitive-behavioral dysfunction (100% of patients), seizures (85% of patients), and AHC (71% of patients). Moreover, they further expand the evolving allelic spectrum of these disorders by identifying 3 novel mutations.
Funding: No funding was received for this abstract because it is a retrospective study.