Structural Connectivity Driven Stereoelectroencephalography (SEEG) Electrode Targeting in Suspected Pseudotemporal and Temporal Plus Epilepsy
Abstract number :
2.168
Submission category :
5. Neuro Imaging / 5A. Structural Imaging
Year :
2018
Submission ID :
501193
Source :
www.aesnet.org
Presentation date :
12/2/2018 4:04:48 PM
Published date :
Nov 5, 2018, 18:00 PM
Authors :
Martina Della Costanza, Università Politecnica delle Marche, Azienza Ospedaliera Umberto I; Vejay N. Vakharia, University College London; Kuo Li, University College London; Matteo Mancini, Wellcome EPSRC Centre for Interventional and Surgical Science
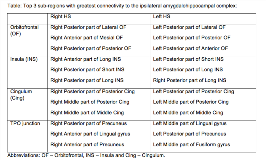
Rationale: One third of patients with drug resistant mesial focal temporal lobe epilepsy (MTLE) fail to achieve long-term seizure freedom following temporal lobe resections. Reasons for failure may include ictal onset outside the temporal lobe (TL), termed “pseudotemporal lobe epilepsy” (pTLE), with spread from strongly connected neighboring areas or temporal plus (TL+) epilepsy, when the epileptogenic zone primarily involves the temporal lobe and also extends to neighboring regions. In such cases the perisylvian and orbitofrontal cortices, cingulum and temporo-parieto-occipital junction may be implicated. Stereoelectroencephalography (SEEG) is a procedure in which electrodes are stereotactically placed within predefined brain regions to delineate the SOZ and allows evaluation of deep anatomical structures adjacent to the TL. SEEG electrode contacts sample from a core radius of 3-5 mm. It is unclear which sub-regions of target structures should be preferentially implanted to optimally detect the network involved in seizure onset and rapid propagation. Using normalized average group templates of structural connectivity from patients with hippocampal sclerosis (HS), we determine the greatest connectivity to critical sub-regions and based upon this propose optimal locations for SEEG targeting. Methods: Twelve patients with HS (6 right) that had undergone SEEG and pre-operative diffusion imaging were identified from a prospectively maintained database. Whole brain connectomes with 10 million tracts were generated using cortical seed regions derived from whole brain GIF parcellations. Normalized group templates were generated separately for right and left HS patients. Orbitofrontal cortex, insula, cingulum and temporo-parietal-occipital junction (supramarginal gyrus, angular gyrus, precuneus, fusiform gyrus, lingual gyrus) were segmented into surgically targetable subregions. All subregions had similar volumes. Connectivity of the amygdalohippocampal complex was defined based on the number of streamlines terminating in the subregions of interest. Results: See the table showing the top 3 sub-regions with greatest connectivity to the ipsilateral amygdalohippocampal complex. Conclusions: Using whole brain connectomes we determine surgically feasible targets in sub-regions based on greatest connectivity to the amygdalohippocampal complex. We propose that SEEG targeting utilizing computer-assisted planning may improve the understanding of the overall network connectivity in order to enhance the diagnostic utility of the SEEG implantation. SEEG electrode placement within structures associated with pTLE and TL+ may aid in delineating the SOZ if the correct sub-regions are targeted. This should be evaluated prospectively. Funding: NIHR UCLH/UCL Biomedical Research Centre, senior investigator schemes, NIH NINDS U01 NS090407and Wellcome Trust (WT106882) / Wellcome/EPSRC (203145Z/16/Z).
.tmb-.png?Culture=en&sfvrsn=4f01f106_0)