Comparison of Treatment Outcomes at 24 Months After Monotherapy With Single-Source Bioequivalent Generic Formulations of Lamotrigine and Levetiracetam in Patients with Newly Diagnosed Focal Epilepsy
Abstract number :
2.147
Submission category :
4. Clinical Epilepsy / 4C. Clinical Treatments
Year :
2018
Submission ID :
501144
Source :
www.aesnet.org
Presentation date :
12/2/2018 4:04:48 PM
Published date :
Nov 5, 2018, 18:00 PM
Authors :
Sirichai Chayasirisobhon, Kaiser Permanente; Suresh Gurbani, Kaiser Permanente; Aditya Gurbani, University of Southern California; Usha Vaghasia, Kaiser Pemanente; Erika Pietzsch, Kaiser Permanente; and Benjamin Spurgeon, Kaiser Permanente
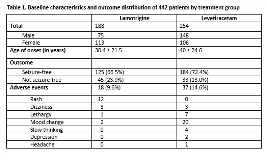
Rationale: Kaiser Permanente advocates use of bioequivalent generic for brand name antiepileptic drugs (AEDs). Such substitution was linked by anecdotal reports to loss of seizure control. The SANAD trial concluded that Lamotrigine (LTG) was the drug of first choice for focal epilepsy1. The LaLiMo trial found no significant difference with regards to efficacy of LTG and levetiracetam (LEV) in the management of newly diagnosed epilepsy2. A systematic review and meta-analysis of generic vs brand name AEDs showed little evidence-based rationale to challenge the implementation of generic substitution for AEDs3. However, most of these studies were of short duration. We compared efficacy and tolerability of generic LTG and LEV formulations at 24-month study period in the treatment of newly diagnosed focal epilepsies. Methods: This was a retrospective data analysis study of 442 consecutive AED naïve patients with new onset focal epilepsy undergoing treatment with either LTG or LEV between 2006 and 2012. To allow for slow titration rate needed to minimize the risk of life threatening events (Stevens Johnson syndrome and toxic epidermal necrolysis) during LTG therapy initiation, AED dosing regimen adjustments were permitted during first 6 months. Chi-square and p-value at < 0.05 were calculated to determine efficacy (seizure freedom) and tolerability of LTG and LEV at 24 months. Results: A total of 188 patients were started on LTG and 254 on LEV. During the 24-month study period, 125 (66.5%) patients in LTG group continued to remain seizure free, 45 (23.9%) were drug failures, and 18 (9.6%) dropped out due to adverse events while in the LEV group, seizure freedom was maintained in 184 (72.4%), with 33 (13.0%) drug failures and 37 (14.6%) drop outs due to adverse events, with p-value for seizure freedom 0.18 and for tolerability 0.12, both not significant at p < 0.05. There were no fatalities or hospital admissions due to adverse events. Conclusions: Single-source bioequivalent generic LTG and LEV formulations were comparable for seizure freedom rates and tolerability at 24 months treatment period. These response rates are comparable to those reported in literature for brand name AEDs 1,2,3.References:Marson AG, Al-Kharusi AM, Alwaidh M, et al; SANAD Study group. The SANAD study of effectiveness of carbamazepine, gabapentin, lamotrigine, oxcarbazepine, or topiramate for treatment of partial epilepsy: an unblinded randomized controlled trial. Lancet. 2007;369:1000-1015.Rosenow F, Schade-Brittinger C, Burchardi N, et al; LaLiMo study group. The LaLiMo Trial: lamotrigine compared with levetiracetam in the initial 26 weeks of monotherapy for focal and generalized epilepsy-an open-label, prospective, randomized controlled multicenter study. JNNP. 2012;83(11):1093-1098.Kesselheim AS, Stedman MR, Bubrick EJ, et al. Seizure outcomes following use of generic vs. brand-name antiepileptic drugs: A systematic review and meta-analysis. Drugs. 2010;70(5):605-621. Funding: None
.tmb-.png?Culture=en&sfvrsn=3d75f4a0_0)