EFFECT OF ADJUNCTIVE RUFINAMIDE IN PEDIATRIC PATIENTS WITH INADEQUATELY CONTROLLED LENNOX-GASTAUT SYNDROME: INTERIM PHARMACOKINETIC AND SAFETY RESULTS FROM STUDY 303
Abstract number :
2.410
Submission category :
Year :
2014
Submission ID :
1868962
Source :
www.aesnet.org
Presentation date :
12/6/2014 12:00:00 AM
Published date :
Dec 4, 2014, 06:00 AM
Authors :
Jose Ferreira, Alexis Arzimanoglou, Shannon Mendes, Betsy Williams, David Critchley, Edgar Schuck, Dinesh Kumar and Francesco Bibbiani
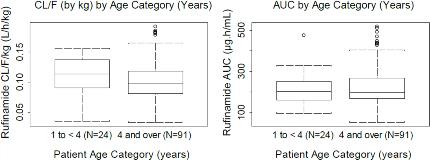
Rationale: Study 303 is a Phase III, open-label, 2-year study of adjunctive rufinamide (RFM) (oral suspension) treatment in pediatric subjects ≥1 to <4years with inadequately controlled LGS. This interim subanalysis reports the pharmacokinetics (PK) and 6-month safety of RFM compared to any other investigator-chosen antiepileptic drug (AED) as add-on treatments in this pediatric population, while the cognitive endpoints will be reported at the end of the 2-year study. Methods: Subjects were randomized 2:1 to either RFM or an approved AED chosen by the investigator as add-on to an existing regimen of 1-3 AEDs. RFM was administered at 10-mg/kg/day (2 equally divided doses), increased in 10-mg/kg/day increments every 3 days to 40-mg/kg/day, and then increased by 5-mg/kg/day to 45-mg/kg/day (target dose). Other AEDs were administered according to the investigator's usual practice. Study phases consisted of pre-randomization (screening period + baseline visit=8 wks) and randomization (titration period + maintenance period=106 wks; taper period=2 wks). The population PK analysis included plasma RFM concentrations from LGS subjects aged ≥1 to <4 (n=24, Study 303) and ≥4years (n=65, Study 022; n=26, Study 304). Safety endpoints for Study 303 included treatment-emergent and serious adverse events (TEAEs; SAEs) using Medical Dictionary for Regulatory Activities (MedDRA) standardized terms, clinical laboratory parameters, vital signs, and 12-lead ECG. Results: The PK of RFM was dose-independent. The estimated basal apparent clearance (CL/F) from the PK model was 2.19L/h; it was found to increase significantly as a function of body weight (estimated exponent weight effect on CL/F=0.831; 95% CI: 0.704-0.958). Co-administration of valproic acid significantly decreased the CL/F of RFM with a slope of -0.496 (95% CI: -0.704--0.288); this effect confirms what has already been observed in ≥4years. CL/F was not significantly affected by other concomitant AEDs or by age, gender, race, hepatic function, or renal function. Clearance and exposure to RFM in subjects aged ≥1 to <4years was comparable to that in subjects ≥4years (Fig 1). TEAE incidence in Study 303 was similar between the RFM (88.0%) and the any-other-AED group (81.8%), with most events considered mild or moderate (Table 1). SAEs occurred in 6 (24.0%) and 3 (27.3%) subjects in the RFM and any-other-AED groups, respectively. There were no clinically important mean changes in laboratory values, vital signs, or ECG. Conclusions: Results from the Study 303 interim analysis showed that the PK of RFM in subjects ≥1 to <4years is comparable to that from previous analyses in subjects ≥4years with LGS. No adjustments are necessary to the body-weight-based dosing for RFM in subjects ≥1 to <4years of age. RFM was safe and well tolerated in these pediatric subjects. Support: Eisai Inc.