Efficacy, Safety and Tolerability of Eslicarbazepine acetate in Post-Stroke Epilepsy: Real-World Evidence from the Euro-Esli Study
Abstract number :
2.465
Submission category :
7. Antiepileptic Drugs / 7B. Clinical Trials
Year :
2018
Submission ID :
554327
Source :
www.aesnet.org
Presentation date :
12/2/2018 4:04:48 PM
Published date :
Nov 5, 2018, 18:00 PM
Authors :
João Chaves, Hospital Santo António - Centro Hospitalar do Porto; Francisco Sales, Centro Hospitalar e Universitário de Coimbra; Vicente Villanueva, Hospital Universitario y Politécnico La Fe; Rob McMurray, Eisai Europe Ltd; Rui Lourei
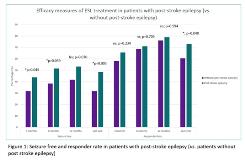
Rationale: Cerebrovascular disease may account for almost 50% of new cases of epilepsy in patients older than age 65. A recent systematic review with network metanalysis of randomized controlled trials (RCT) of antiepileptic drugs (AED) for the treatment of post-stroke epilepsy was inconclusive due to the small number of trials (n=2) included. Our aim was to conduct an exploratory subgroup analysis of data from patients included in Euro-Esli study to evaluate the efficacy, safety and tolerability of eslicarbazepine acetate (ESL) in post-stroke epilepsy. Methods: The Euro-Esli study was an exploratory, pooled analysis of data from 14 European clinical practice studies, that included 2079 patients. Responder rate (≥50% seizure frequency reduction from baseline) and seizure freedom rate (seizure freedom at least since prior visit) were assessed after 3, 6 and 12 months of ESL treatment, and at last visit, as many patients were followed for >1 year. Adverse events (AEs) and AEs leading to ESL discontinuation were assessed throughout follow-up. Subgroup analyses were conducted for patients who had vascular vs. non-vascular aetiology of epilepsy. Results: This population subanalysis included 1656 patients, of whom 76 (4.6%) had post-stroke epilepsy and 60.5% were male (n=46). Patients with post-stroke epilepsy were older (60.4 vs. 42.9 years; pvs. 23.7 years; pvs. 19.4 years; pvs. 13.8 seizures/month; pvs. 4.4; pvs. 1.1; pvs. 31.7%; p=0.003); than in patients without post-stroke epilepsy as was the percentage of responders (72.9% vs. 60.6%; p=0.040). AEs (36.0% vs. 35.8%; p=0.966) and withdrawal due to AEs (6.8% vs. 14.5%; p=0.063) differences between patients with post-stroke epilepsy and without post-stroke epilepsy were not statistically significant. The most common AEs were somnolence, dizziness, instability and fatigue. As of the 12-month follow up visit, the mean time in treatment with ESL in patients with post-stroke epilepsy was 11.2 months and in patients without post-stroke epilepsy was 10.2 months (p=0.023; Log Rank test). The percentage of patients who discontinued for any reason, at 12 months, was higher in patients without post-stroke epilepsy, than in patients with epilepsy with vascular aetiology (22.6% vs. 12.2%; p=0.035). Conclusions: In this post-hoc analysis of data pooled from a large cohort of patients treated in the real-world clinical setting in Europe, ESL was effective and well tolerated in a small subgroup of patients with post-stroke epilepsy. Funding: Study subanalysis supported by Bial
.tmb-.jpg?Culture=en&sfvrsn=90419d89_0)