Preliminary Evidence of a Predictive Clinical Biomarker in PCDH19-Related Epilepsy: Significant Treatment Effect of Ganaxolone In Biomarker-Positive Patients
Abstract number :
2.251
Submission category :
7. Antiepileptic Drugs / 7B. Clinical Trials
Year :
2018
Submission ID :
502507
Source :
www.aesnet.org
Presentation date :
12/2/2018 4:04:48 PM
Published date :
Nov 5, 2018, 18:00 PM
Authors :
Joseph Sullivan, University of California, San Francisco Benioff Children’s Hospital; Nicola Specchio, Bambino Gesù Children Hospital; Michael G. Chez, Sutter Neuroscience Institute; Graziano Pinna, University of Illinois at Chicago; Andrea Loc
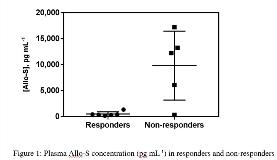
Rationale: The use of predictive biomarkers to guide personalized disease treatment has revolutionized the field of medicine. Although biomarker discoveries have made substantial inroads in therapeutic areas such as oncology, minimal progress has been made in the field of epilepsy. A biomarker that could differentiate a drug responder from a non-responder would have a profound impact on patients with epilepsy. Rare pediatric epilepsies represent conditions of unmet medical need. Mutation of the protocadherin-19 (PCDH19) gene leads to a rare form of pediatric epilepsy. PCDH19-related epilepsy primarily affects females with early-onset seizures (4-48 months) that frequently occur in clusters. Individuals with pathogenic variants in PCDH19 had been observed to have decreased endogenous allopregnanolone levels compared to age-matched controls. Allopregnanolone is a neurosteroid that acts as a positive allosteric modulator of the GABAA receptor, and it has been shown in some settings to have anticonvulsant and anxiolytic effects. The observation of low allopregnanolone levels in patients with PCDH19 variants provided the scientific rationale for an open-label Phase 2 study of ganaxolone, an allopregnanolone analog, for the treatment of PCDH19-related epilepsy. Herein we report for the first time preliminary evidence of a predictive clinical biomarker in PCDH19-related epilepsy and the justification for an innovative future clinical trial design. Methods: Individuals (n=11) with a confirmed PCDH19 mutation were enrolled in 2015 at six centers in the U.S. and Italy to receive oral ganaxolone up to 1,800 mg daily for up to 26 weeks. Seizure frequency change (%) was assessed as the primary endpoint and a responder was defined as having a = 25% decrease in seizure rate. Plasma neurosteroid levels were collected pre-treatment and quantified using a previously published GC/MS method. Results: The median change in 28-day seizure frequency (all seizure types) from baseline for all-comers (n=11) was a decrease of 26%. In this group, average plasma allopregnanolone-sulfate (Allo-S) concentration was 4,741 pg mL-1 (median=433 pg mL-1). The responder analysis and correlation with Allo-S demonstrated two discrete populations. Responders (n=6) and non-responders (n=5) had plasma Allo-S concentrations of 501 ± 430 pg mL-1 and 9,829 ± 6,638 pg mL-1, respectively (mean ± SD, p=0.05, Mann-Whitney) (Figure 1). Retrospective analysis of biomarker+ (n=7, Allo-S < 2,500 pg mL-1) versus biomarker- (n=4, Allo-S > 2,500 pg mL-1) subjects yielded median % change seizure rates of -53.9% and 247%, respectively (p=0.006, Mann-Whitney). Further, the biomarker+ group significantly improved (p=0.02, Wilcoxon) whereas the biomarker- group did not significantly deteriorate (p=0.25, Wilcoxon) when comparing seizure frequency at 6 months to baseline. Conclusions: Here we present early evidence of a plasma Allo-S biomarker that may be used to predict seizure response in patients with PCDH19-related epilepsy when treated with ganaxolone. Post-treatment review of baseline plasma neurosteroid levels in patients with PCDH19-related epilepsy revealed a significant association between these levels and response to ganaxolone treatment. A 54% reduction in seizure rate in biomarker+ patients is medically notable in this highly treatment refractory patient population. Despite the need for future research to understand the neurobiological relevance of these findings, this discovery presents an opportunity to conduct a biomarker-stratified clinical trial in PCDH19 with ganaxolone. Funding: Marinus Pharmaceuticals
.tmb-.jpg?Culture=en&sfvrsn=32f6b44a_0)