A Conditional Neuronal Emx1cre Nprl2 Model of Spontaneous Seizures
Abstract number :
1.020
Submission category :
1. Basic Mechanisms / 1B. Epileptogenesis of genetic epilepsies
Year :
2018
Submission ID :
496317
Source :
www.aesnet.org
Presentation date :
12/1/2018 6:00:00 PM
Published date :
Nov 5, 2018, 18:00 PM
Authors :
Brianne Dentel, University of Texas Southwestern Medical Center and Peter Tsai, University of Texas Southwestern Medical Center
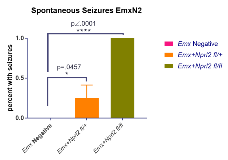
Rationale: Mutations in the mechanistic Target Of Rapamycin (mTOR) pathway are a major cause of genetic epilepsy. GTPase-activating protein [GAP] activity towards Rags1 complex (GATOR1 complex) is a key negative regulator of the mTOR pathway. Mutations in the GATOR1 subunits NPRL2 (nitrogen protease regulator like 2), NPRL3 (nitrogen permease regulator like 3), and DEPDC5 (DEP Domain Containing 5) have been identified in genetic epilepsies. To understand the contribution of this complex to epilepsy, it is therefore critical to develop mouse models that capture the human clinical picture of focal epilepsies. Specifically, two of the most common clinical findings are spontaneous seizures and cortical patterning abnormalities. Methods: To investigate the role of GATOR1 in epilepsy we have generated a conditional brain specific knockout of Nprl2. We utilized Emx1cre as Emx1 is expressed in 80% of excitatory neurons and some glia in the forebrain and hippocampus.We first confirmed disruption of GATOR1 negative regulation of mTOR function. Mice were monitored for spontaneous seizure activity, and survival curves were attained. Brains were also stained with Cux1, Foxp1, Tbr1, FoxP2 to characterize cortical layering. Results: We have observed significant spontaneous seizure activity upon handling in both heterozygous (25%, n=8) and homozygous (100%, n=9) Emx1creNprl2 mutant mice. Homozygous Emx1creNprl2 mutants die by p21 while no decreased survival is noted in littermate controls. Underlying pathology shows that cortical width increases in mutant mice. Control 922.4um ± 35.32um, n=3 mutant mice 1144 um ± 59.48 um, n=3, p=.0330 using an unpaired two tailed t-test. Staining revealed preliminary evidence for altered and wider cortical layer patterning. Conclusions: The Emx1cre Nprl2 mouse model of epilepsy has spontaneous seizures and homozygous mutants have decreased survival. The pathological effects of the deletion of Nprl2 in the forebrain and hippocampus displays increased cortical thickness, indicating a developmental abnormality relating to susceptibility to seizures. This is a valuable model of genetic epilepsy in mice where the heterozygous mice do display spontaneous seizures, and cortical pathology is abnormal in homozygous mutant mice. Because these mice can recapitulate spontaneous seizures and other pathology similar to the human condition, such a model will provide an important tool to evaluate the mechanisms of GATOR1 epilepsy and provide a way to evaluate treatment modalities that could be relevant to human clinical epilepsy. Funding: The Medical Scientist Training Program training grant from the National Institute of General Medical Sciences of the National Institutes of Health
.tmb-.png?Culture=en&sfvrsn=9a590507_0)