Direct Neurotransmitter Activation of Voltage-Gated Potassium Channels
Abstract number :
1.069
Submission category :
1. Basic Mechanisms / 1F. Other
Year :
2018
Submission ID :
497664
Source :
www.aesnet.org
Presentation date :
12/1/2018 6:00:00 PM
Published date :
Nov 5, 2018, 18:00 PM
Authors :
Geoffrey W. Abbott, University of California - Irvine; Maria Papanikolaou, University of California - Irvine; and Rian Manville, University of California - Irvine
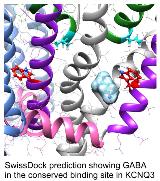
Rationale: Voltage-gated potassium channels KCNQ2-5 generate the M-current, which controls neuronal excitability. Inherited mutations in the genes encoding these subunits cause epilepsy syndromes. KCNQ2-5 channels, and in particular KCNQ2/3 heteromers, are a target for some anticonvulsants, including retigabine. KCNQ2-5 subunits each harbor a high-affinity retigabine binding pocket containing an essential tryptophan (W265 in human KCNQ3) conserved for >500 million years, yet lacking a known physiological function. Methods: Phylogenetic analysis, electrostatic potential mapping, in silico docking, electrophysiology and radioligand binding assays were used to test the hypothesis that the conserved binding pocket in KCNQ2-5 subunits evolved to accommodate endogenous ligands. Results: Our experiments revealed that the conserved retigabine binding pocket evolved to bind endogenous neurotransmitters including ?-aminobutyric acid (GABA), which directly activated KCNQ5 and KCNQ3 via W265. GABA, and endogenous metabolites such as ß-hydroxybutyric acid (BHB) and ?-amino-ß-hydroxybutyric acid (GABOB), competitively and differentially shift the voltage dependence of KCNQ3 activation. Conclusions: Our results uncover a new paradigm: chemosensing of the neurotransmitter/metabolite landscape by voltage-gated ion channels to regulate channel activity and cellular excitability. Funding: This study was supported by the US National Institutes of Health (GM115189 to GWA).