Long-Term Effectiveness of Adjunctive Brivaracetam in Children With Epilepsy: Pooled Analysis of Data From Two Open-Label Trials
Abstract number :
1.293
Submission category :
7. Antiepileptic Drugs / 7B. Clinical Trials
Year :
2018
Submission ID :
497210
Source :
www.aesnet.org
Presentation date :
12/1/2018 6:00:00 PM
Published date :
Nov 5, 2018, 18:00 PM
Authors :
Teresa Gasalla, UCB Pharma; Melinda Martin, UCB Pharma; Sami Elmoufti, UCB Pharma; Vincent Badalamenti, UCB Pharma; Jan-Peer Elshoff, UCB Pharma; and Anup D. Patel, Nationwide Children's Hospital
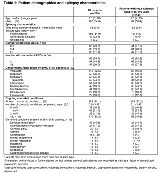
Rationale: In the US, approval of brivaracetam (BRV) for treatment of children (≥4 years) with focal (partial-onset) seizures was based on extrapolation of efficacy data from adult trials. Efficacy and effectiveness of BRV in children have not been evaluated in randomized placebo-controlled trials. This post hoc analysis assessed the long-term effectiveness of BRV in children (aged ≥4 to <16 years) with focal seizures, based on data from open-label safety, tolerability, and pharmacokinetic trials. Methods: N01263 (NCT00422422) was an open-label trial of adjunctive BRV in children (aged ≥1 month to <16 years) with epilepsy, uncontrolled by 1-3 concomitant antiepileptic drugs (AEDs). BRV dose was up titrated over 3 weeks (patients aged ≥8 years: 0.4, 0.8, 1.6 mg/kg bid; patients aged <8 years: 0.5, 1.0, 2.0 mg/kg bid). Patients who completed the 3-week dose-escalation period could continue on BRV in an open-label extension trial (N01266; NCT01364597), which also enrolled new patients (aged ≥4 to <17 years) with uncontrolled focal seizures. After N01266 dose-adjustment phase, patients received BRV at a dose of 1-5 mg/kg/day (maximum 200 mg/day). This pooled interim analysis (cut-off: 15 March 2017) was conducted for all patients, and for patients with focal seizures aged ≥4 to <16 years (focal 4-16 subgroup). Kaplan-Meier estimated 12- and 24-month retention rates were calculated from time of first exposure to BRV. Results: Data from 219 patients were analyzed. 186 (84.9%) had focal seizures, of whom 149 were aged ≥4 to <16 years and included in the focal 4-16 subgroup (Table 1). At the cut-off date, 53.9% of patients remained on BRV, 44.3% had discontinued, and 1.8% had chosen not to enter N01266 (Table 2). The most common reasons for discontinuation (≥8%) were lack of efficacy (15.1%), adverse events (11.9%), and patient choice (9.1%) (focal 4-16 subgroup: lack of efficacy [13.4%]; patient choice [8.7%]; adverse events [8.1%]) (Table 2). Overall, total exposure to BRV was 464.6 patient years (focal 4-16 subgroup: 299.4 years); 68.0% of patients had ≥12 months and 51.6% had ≥24 months of treatment (focal 4-16 subgroup: 71.8%; 52.3%). Kaplan-Meier estimated 12- and 24-month retention rates for all patients were 68.0% (95% CI: 61.4, 73.8) and 60.3% (53.4, 66.5), respectively. For the focal 4-16 subgroup, Kaplan-Meier estimated 12- and 24-month retention rates were 71.8% (63.8, 78.3), and 64.5% (56.1, 71.6). Treatment-emergent adverse events (TEAEs) were reported by 94.5% of all patients, most commonly (≥ 20% of patients) nasopharyngitis, pyrexia, convulsion, vomiting and pharyngitis (Table 2). The most common (≥ 5% of patients) drug-related TEAEs (judged by investigator) were somnolence (6.8%), and decreased appetite (6.4%). TEAEs leading to discontinuation in ≥1% of patients were aggression (1.4%) and suicidal ideation (1.4%). Conclusions: This post hoc analysis illustrates the effectiveness of BRV in the long-term treatment of focal epilepsy in children aged ≥4 to <16 years. BRV was generally well-tolerated, with few discontinuations due to TEAEs. Funding: UCB Pharma-sponsored
.tmb-.png?Culture=en&sfvrsn=1c136da4_0)