Pediatric Experience With Responsive Neurostimulation
Abstract number :
1.356
Submission category :
9. Surgery / 9B. Pediatrics
Year :
2018
Submission ID :
507123
Source :
www.aesnet.org
Presentation date :
12/1/2018 6:00:00 PM
Published date :
Nov 5, 2018, 18:00 PM
Authors :
Malgosia Kokoszka, Mount Sinai Health System; Maite La Vega-Talbott, Mount Sinai Health System; Patricia McGoldrick, Icahn School of Medicine at Mount Sinai; Madeline Fields, Icahn School of Medicine at Mount Sinai; Lara Marcuse, Icahn School of Medicine
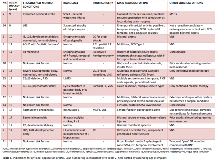
Rationale: To report clinical decision-making, outcomes and complications in 16 consecutive pediatric patients with medically refractory epilepsy who were treated off label with responsive neurostimulation (RNS) Methods: Review of medical records Results: Sixteen pediatric patients (8M, 8F; ages 9-17, mean age 14±3 years) with medically refractory seizures were treated off label with RNS therapy between September 2015 and March 2018. Six of the 16 patients had previous resective and/or disconnective procedures, in 2 cases combined with vagus nerve stimulation (VNS), 1 patient had multiple subpial transections (MST’s) and VNS therapy, and in 9 of the patients, RNS was the initial surgical intervention. The patients were not considered candidates for traditional resective surgery except for 1 patient (Case 11) with unilateral multifocal epilepsy where a partial resection was possible (see Table 1. Indications for off label use of RNS).At a mean follow up of 14±9 months (median 14 months, range 2-32 months), 9 of the patients experienced >50% seizure reduction, including 4 patients with >90% seizure reduction and 1 who only had a single seizure in the 19 months since surgery. Additionally, 3 patients experienced seizure reduction at a rate of 50% or less, 4 patients had no appreciable change in seizure frequency, and 1 patient’s seizures became worse. Notably, 10 patients also experienced a reduction in seizure severity/duration and/or postictal recovery times and the majority of patients had additional functional/quality of life/diagnostic benefits of RNS therapy (see Table 2. Current seizure outcomes). All patients continue to have detection and stimulation parameters evaluated monthly and modified based on clinical outcomes. One patient (Case 5) had implant-related complications which included superficial infection with explant of 2 electrodes and lead fracture that was associated with a temporary increase in seizure frequency and required surgical replacement. Conclusions: Early results of adjunctive RNS therapy in our pediatric series are promising, with the majority of patients experiencing improvement ranging from reduction in seizure duration to seizure freedom. The complications associated with 1 of our cases were not deemed to be specifically due to pediatric age and we found no indication that complication rate in children should be different from that established in adult patients. As is the case in adults, the benefits of RNS observed in our series tend to increase with time and continued therapy, making it particularly suited for treatment of childhood epilepsies. Strong indications for off-label use of this surgically implanted device need to be present, but the reversible and modifiable nature of RNS makes it an appealing option in children with difficult to treat epilepsy who have low potential for seizure freedom with resection or have unresectable seizure foci. Funding: This project was supported by the Department of Neurosurgery, Mount Sinai Health System.
.tmb-.png?Culture=en&sfvrsn=18ac926e_0)