Prediction of Postoperative Deficit Using an Improved Diffusion Tensor Imaging Maximum a posteriori Probability Analysis in Pediatric Epilepsy Surgery
Abstract number :
2.327
Submission category :
9. Surgery / 9B. Pediatrics
Year :
2018
Submission ID :
500314
Source :
www.aesnet.org
Presentation date :
12/2/2018 4:04:48 PM
Published date :
Nov 5, 2018, 18:00 PM
Authors :
Jeong-Won Jeong, Children’s Hospital of Michigan, Wayne State University, Detroit Medical Center; Min-Hee Lee, Children's Hospital of Michigan, Wayne State University, Detroit Medical Center; Yasuo Nakai, Children’s Hospital of Michigan, Wayne
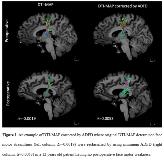
Rationale: We previously reported that diffusion tensor imaging-based maximum a posteriori probability (DTI-MAP) method can localize individual streamline fibers of primary motor and language pathways with accuracy of 76-82%, as compared with the results of gold-standard electrical stimulation mapping. However, the equal class prior assumption incorporated in the DTI-MAP inevitably increases type I and II errors when classifying outliers. Thus, we hypothesized that correction of the original DTI-MAP streamlines by adopting average direct-flip distance (ADFD, mean distance between equally sampled bidirectional fibers) would reduce the errors and increase the accuracy of prediction of eloquent cortex. We determined if ADFD-corrected DTI-MAP can predict the occurrence of postoperative deficit with greater accuracy compared to the conventional clinical variables or the original DTI-MAP. Methods: We retrospectively studied 24 children (age: 11.3±3.8 years) who underwent resection of epileptic foci and had at least a six-month follow-up to evaluate three categories of postoperative deficits, requiring face motor (n=4), hand motor (n=4), or language therapy (n=5). In the resected hemisphere of pre-/post-operative DTI, DTI-MAP identified a total of 8 streamline classes: face motor/hand, motor/Broca’s-Wernicke’s/Broca’s-Premotor/Premotor-Parietal/Wernicke’s-Parietal/Wernicke’s-Premotor/Others. The original DTI-MAP streamlines were reclassified by referring to their minimum ADFDs to the class exemplars (i.e., the most representative class streamlines obtained from healthy controls). That is, for each streamline of the identified class, ADFDs to 8 exemplars were evaluated in standard space, where the class membership of an exemplar having minimum ADFD (<10mm) determined the new membership. Finally, postoperative streamline volume change of the corrected class, ?=(post-pre)/pre-intracranial volume in face motor, hand motor and 5 language classes, was separately analyzed by multivariate logistic regression and receiver operating characteristic analysis in order to investigate if ‘DTI-MAP corrected by ADFD’ would improve the prediction of postoperative deficit outcome in face motor, hand motor, and language. Results: Fig. 1 shows an example showing the efficacy of ADFD on DTI-MAP analysis whose major streamlines were appropriately reclassified from other classes to the correct class, resulting in +? to indicate no postoperative face motor deficit (i.e., -? was evaluated without the correction indicating postoperative deficit). Addition of ‘DTI-MAP corrected by ADFD’ to clinical variables: age, gender, number of antiepileptic drugs, the presence of daily seizure, indeed improved the outcome predictive ability of the logistic regression model, according to the areas under the curve (from 0.65/0.61/0.84 to 1.0/1.0/1.0 for face/hand/language). Meanwhile, addition of ‘original DTI-MAP’ to clinical variables provided smaller areas under the curve (0.97/0.93/1.0 for face/hand/language), supporting that ‘DTI-MAP corrected by ADFD’ outperforms the original DTI-MAP in prediction of postoperative motor deficits (Table 1). Conclusions: The proposed approach may be an effective quantitative imaging tool to predict postoperative deficits noninvasively and accurately in epilepsy presurgical evaluation. Our results suggest that resection of the eloquent areas as determined by DTI-MAP is associated with increased risk of postoperative neurological deficits. Funding: This study was funded by a grant from National Institute of Neurological Disorders and Stroke (R01-NS089659 to JJ).
.tmb-.jpg?Culture=en&sfvrsn=9504ba4d_0)