Time to Onset of Efficacy of Cannabidiol (CBD) During Titration in Patients with Lennox–Gastaut Syndrome (LGS) and Dravet Syndrome (DS) Enrolled in 3 Randomized Controlled Trials
Abstract number :
1.296
Submission category :
7. Antiepileptic Drugs / 7B. Clinical Trials
Year :
2018
Submission ID :
499756
Source :
www.aesnet.org
Presentation date :
12/1/2018 6:00:00 PM
Published date :
Nov 5, 2018, 18:00 PM
Authors :
Michael Privitera, University of Cincinnati Medical Center; Eric Marsh, Children's Hospital of Philadelphia; Maria Mazurkiewicz-Beldzinska, Medical University of Gdansk; Vicente Villanueva, Hospital Universitario y Politécnico La Fe; Kevan VanLanding
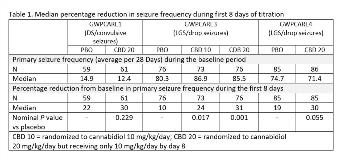
Rationale: Add-on CBD significantly reduced seizures associated with DS and LGS and was generally well tolerated in three phase 3 randomized controlled trials, GWPCARE1 (DS; NCT02091375), GWPCARE3 (LGS; NCT02224560), and GWPCARE4 (LGS; NCT02224690). To better understand the time to onset of efficacy and inform dosing and treatment decisions, we carried out a post hoc analysis of efficacy by cumulative day during the first 14 days of treatment (titration period). Methods: Patients were randomized to receive a plant-derived pharmaceutical formulation of highly purified CBD in oral solution (100 mg/mL) in doses of 10 mg/kg/day (GWPCARE3) or 20 mg/kg/day (all 3 trials) or placebo administered in two divided doses for 14 weeks. Per the titration schedule, patients on both active doses started on 2.5 mg/kg/day CBD and reached 10 mg/kg/day on days 7 and 8 of the titration period; the 20 mg/kg/day arms reached full dose by day 11. Seizures were reported daily using an interactive voice response system. Cumulative frequencies of convulsive seizures (DS) and drop seizures (LGS) were calculated as 28-day averages for each day (ie, including all previous treatment days) of the titration period. Incidence of adverse events (AEs) with onset during the titration period was also assessed. Results: 296 patients were randomized to CBD and 220 to placebo across the three trials. Mean age of patients with DS enrolled in GWPCARE1 was 9 years, and of patients with LGS enrolled in GWPCARE3 and GWPCARE4 was 15 years. Nominal significance for median percentage reductions in frequency of primary seizure types for CBD vs placebo emerged by day 10 in GWPCARE1 (P=0.026), day 6 in GWPCARE3 (P=0.007), and day 9 in GWPCARE4 (P=0.020). Changes in primary seizure frequency during the first 8 days of treatment favored CBD in all 3 trials, with nominal significance for both active arms in GWPCARE3 (Table 1). Of patients with AEs, 60% had their first during the titration period; the difference in overall incidence of AEs between placebo and CBD groups was evident during the titration period but generally to a lesser degree than during the full treatment period; differences between treatment groups for some of the most common AEs were also evident during the titration period (Table 2). Conclusions: A CBD treatment effect began to emerge during the first 8 days of treatment in all 3 trials, although this was only statistically significant in GWPCARE3. Incidence of AEs was greater for CBD than placebo, with over half of patients with AEs having their first occurrence during the titration period. Our findings suggest that the titration schedule used in the GWPCARE trials leads to a significant treatment effect for CBD within 6–10 days during up-titration of CBD. Funding: GW Research Ltd
.tmb-.jpg?Culture=en&sfvrsn=a7067aa0_0)